Clinical Updates
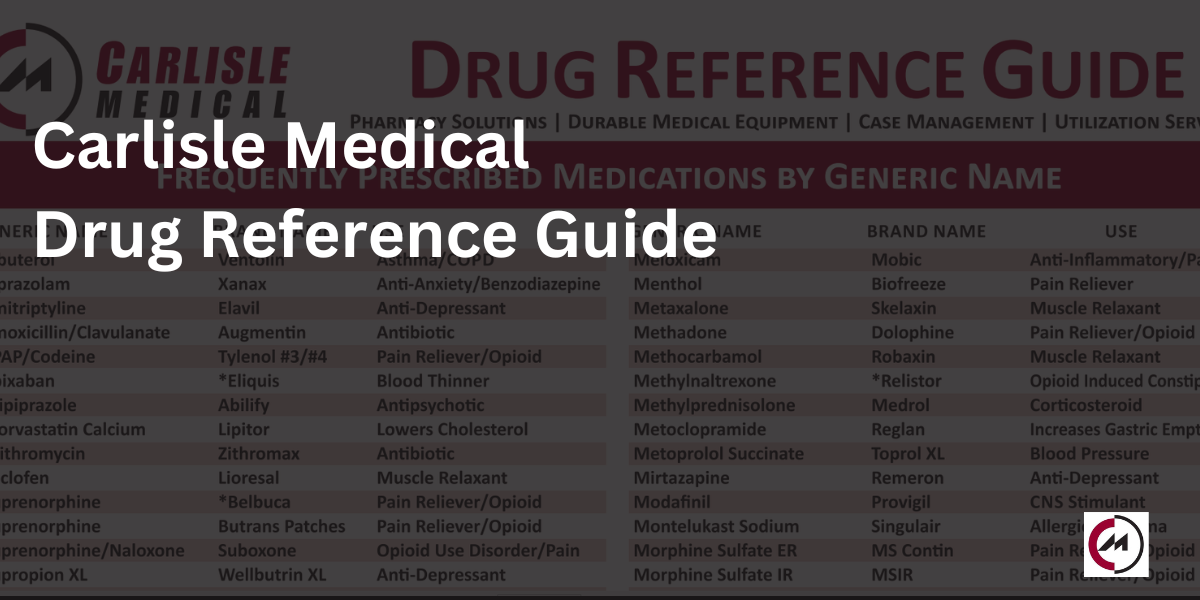

The Carlisle Drug Reference Guide
Carlisle Medical’s Drug Reference Guide has been updated as of July 2024. This guide provides…


The Carlisle Drug Reference Guide
As we enter 2024 we would like to remind you of the Carlisle Drug Reference…


Opvee (Nalmefene) Will Be Released in the Fourth Quarter of 2023
Opvee (Nalmefene) Will Be Released in the Fourth Quarter of 2023 Opvee is indicated for…


Release of Zavzpret In Late July 2023
Release of Zavzpret In Late July 2023 Zavzpret is indicated for the acute treatment of…


The Approval of Over-the-Counter Narcan Nasal Spray
The approval of over-the counter Narcan nasal spray The U.S. Food and Drug Administration approved…


Generic Latuda (Lurasidone Hydrochloride) Is Now Available
The generic formulation of Latuda (Lurasidone Hydrochloride) has been approved by the FDA. Lurasidone Hydrochloride…


Generic Release Of Trokendi XR Is Now Available
Launch of generic Trokendi XR (Topiramate capsule, extended release) Topiramate extended-release capsules are indicated as…


January 2023 Release of the Carlisle Drug Reference Guide
The latest release of the Carlisle Drug Reference Guide provides a list of frequently prescribed…


Generic Release Of Cambia Is Now Available
Launch of generic Cambia (diclofenac potassium powder, for oral solution) Diclofenac potassium powder for oral…


Generic Release Of Pradaxa
The generic formulation of Pradaxa (Dabigatran Etexilate) has been recently released. Dabigatran is used for…


