Clinical Updates

New Medication Release of Symbravo
The FDA recently approved Symbravo® (meloxicam/rizatriptan) for the acute treatment of migraine with or without…

New Medication Release of Tonmya
The FDA has approved Tonmya (cyclobenzaprine HCl sublingual tablet) for the treatment of fibromyalgia in…

Launch of Generic Entresto (sacubitril/valsartan) Tablets
Generic Entresto (sacubitril/valsartan) has recently been approved by the FDA and released in 24mg/26mg, 49mg/51mg…

Nevada Adopts ODG Drug Formulary
Bipartisan legislation makes Nevada the thirteenth state to adopt an ODG tool for workers’ comp…

Launch of Brilinta® (Ticagrelor) Tablets
Generic Brilinta® (Ticagrelor) has been approved by the FDA. Ticagrelor tablets are indicated to reduce…

The Carlisle Drug Reference Guide
This guide provides a list of frequently prescribed medications by brand name, their generic name,…

Launch of Generic Xarelto (Rivaroxaban) Tablets
Generic Xarelto (Rivaroxaban) has recently been approved by the FDA and released in 2.5 mg…

Introducing Journavx™: A New Non-Opioid Pain Relief Medication.
Vertex Pharmaceuticals has introduced Journavx™ (suzetrigine)—a non-opioid medication for moderate-to-severe acute pain in adults. Unlike…
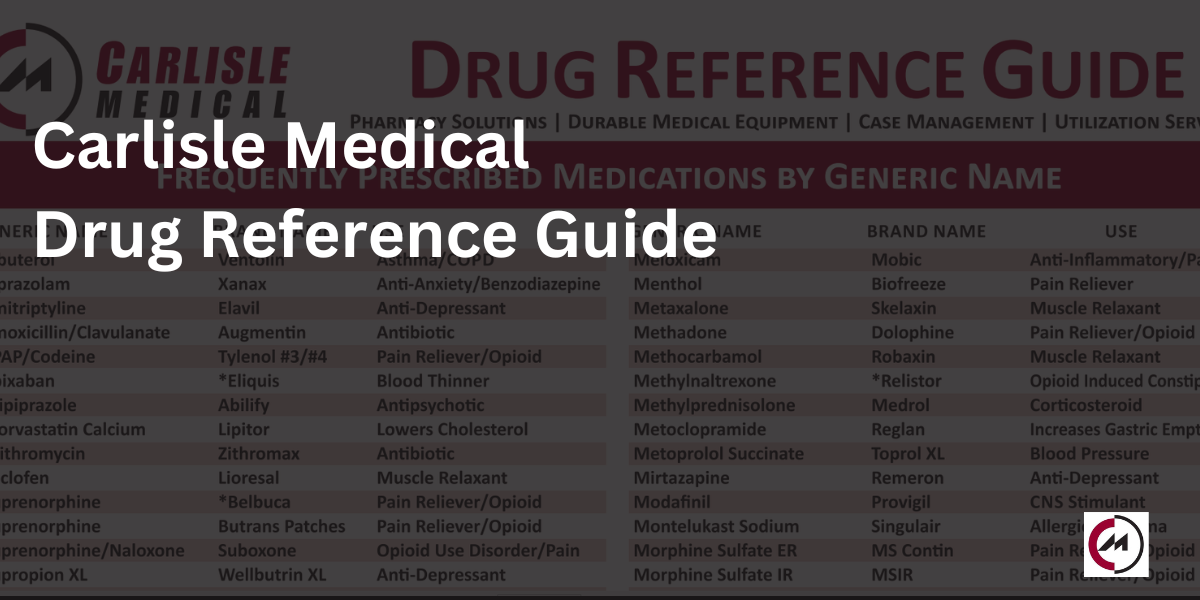
The Carlisle Drug Reference Guide
This guide provides a list of frequently prescribed medications by brand name, their generic name,…

Launch of generic Motegrity (Prucalopride) tablets
Generic Motegrity (Prucalopride) has recently been released and is approved by the FDA. Prucalopride…


